July 25, 2021
Employer:
SGA
Project Role(s)
Lab Planner
Designer
Subject Matter Expert
Description
Lab planning, test fits and schematic designs for multiple projects for various clients. Many are efforts to reposition existing commercial office space to research laboratories.
I wield subject matter expertise and a plethora of analytical, documentation and communication methods to do my work including:BIM
data analysis
visual scripting and automation
rendering and animation
Pre-design / feasibility studies to reposition commercial real estate in a downtown district for the life science market. Notable project challenges include MEP coordination and local bylaws prohibiting rooftop enabling infrastructure to be seen by pedestrians anywhere within the district.
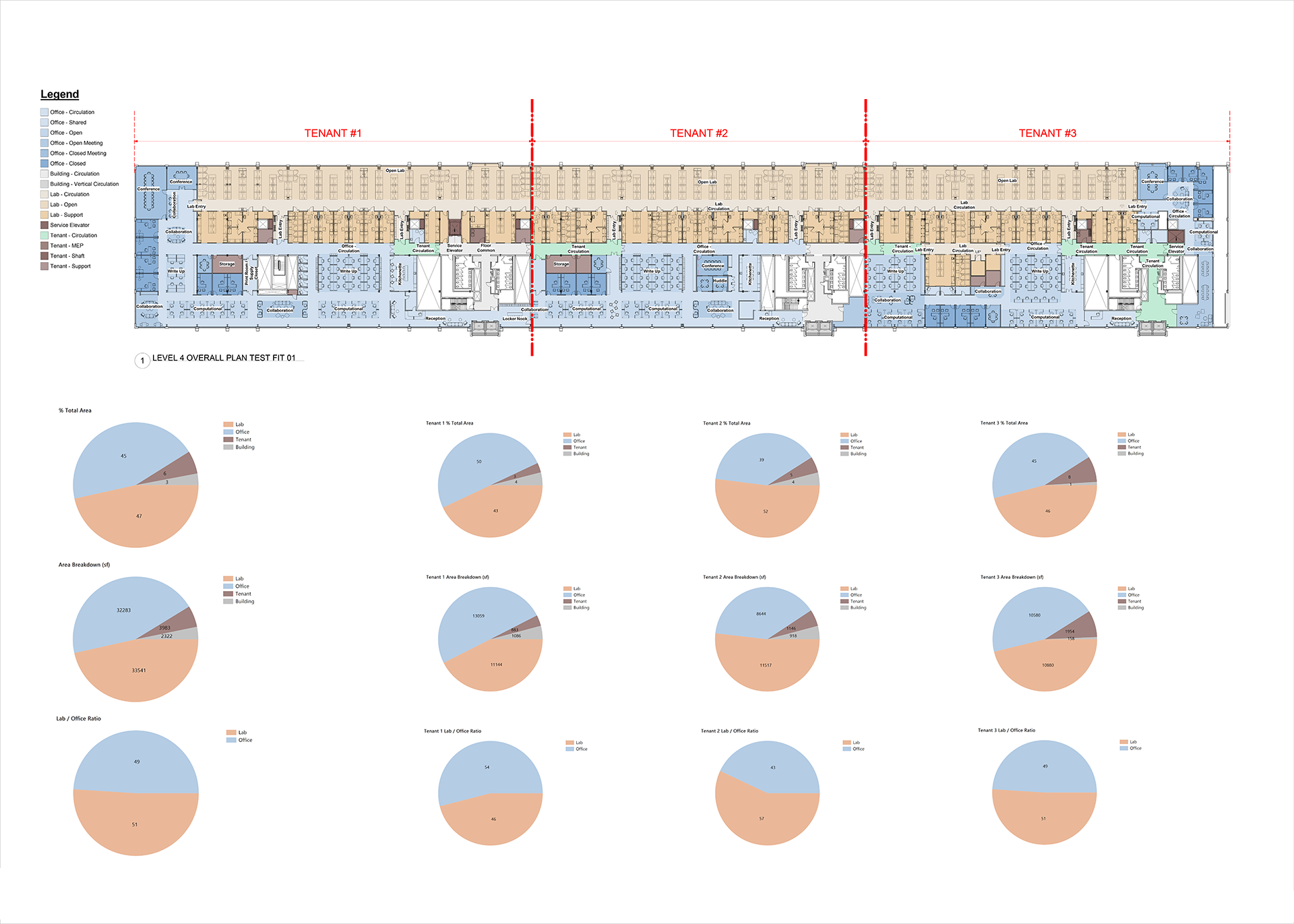
Three test fit design options for a bio medical research occupant including a vivarium. Workflow integrations:
Schedules update automatically noting key design options data including but not limited to:
total lab benches
total offices
total write-up desks
total linear feet of freestanding equipment available.
Pie charts created natively in Revit via Dynamo noting area breakdowns.
Disciplined Flexibility: A 50:50 office-lab floor planned with adaptability in mind. The demising walls between tenant spaces can be adjusted according to tenant needs and market demands. This allows our project teams to deploy capital investment for building enabling infrastructure upgrades with confidence, even though the ultimate make-up and culture of the end user is a moving target. In the face of uncertainty, adaptability is a huge value to our clients and the built environment at large.
Test fitting a cGMP facility in pre-design .Two options for consideration. cGMP facilities must meet stringent FDA guidelines regarding scientifically sound design, processing methods and processing procedures. The terminology in the FDA guidelines are intentionally vague in order to balance insuring the safety and welfare of the end consumer while giving each manufacturer flexibility in how to implement the necessary controls. This means that project teams must invest in adequate expertise to navigate the nuance of designing facilities for current good manufacturing practices.
I believe that a quality design process is one that seeks to make seamless the hand that draws with mind that thinks. I rely on my vast design experience in a variety of science disciplines to inform my models, be it BIM or Excel data, which I also build myself. I leverage technological workflows such as automation to ensure that I am able to do this in a cost (and time) effective manner. Yes, the future is here, where those who think can also produce (and those who produce can also think).
My work is not precious, but time is. Sometimes there is an obvious approach to make a design work within an optimal range. Other times the approach could vary depending on the existing conditions and values of the user. In these situations I’m not shy about exploring multiple approaches in order to help the project team interrogate what trade-offs are the right mix in order for the end user to have a space they feel they can be their unique and best selves in order to produce their best work.
Schematic design of a half floor below and full floor above, repositioning an office building into a mixed office / makerspace for a healthcare diagnostics instrument manufacturer. Notable program elements include clean rooms up to ISO 5 and associated quality control and R&D facilities, as well as office headquarters.